肿瘤坏死因子受体相关因子2重组兔单抗
Rrmab?兔单抗
货号:bsm-62996R
产品详情
相关标记
相关产品
相关文献
常见问题
概述
产品编号
bsm-62996R
产品类型
重组兔单抗、mIHC精品抗体
英文名称
TRAF2 Recombinant Rabbit mAb
中文名称
肿瘤坏死因子受体相关因子2重组兔单抗
英文别名
MGC:45012; RNF117; TRAP; TRAP3; TRAF2_HUMAN; TRAF2; E3 ubiquitin-protein ligase TRAF2; RING-type E3 ubiquitin transferase TRAF2; Tumor necrosis factor type 2 receptor-associated protein 3; 2.3.2.27; TRAF2_MOUSE;
抗体来源
Rabbit
免疫原
A synthesized peptide derived from human TRAF2: 1-28
亚型
IgG
性状
Liquid
纯化方法
affinity purified by Protein A
克隆类型
Recombinant
克隆号
15A5
理论分子量
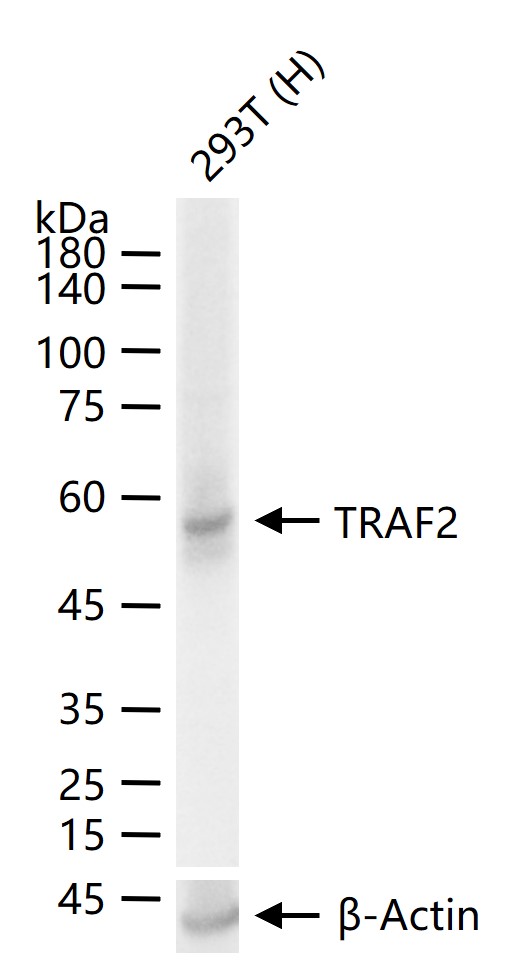
56 kDa
检测分子量
56 kDa
储存液
10mM phosphate buffered saline(pH 7.4) with 150mM sodium chloride, 0.05% BSA, 0.02% Proclin300 and 50% glycerol.
研究领域
Cancer > Growth factors > TNF
Cardiovascular > Atherosclerosis > Vascular Inflammation > Inflammatory mediators
Cell Biology > Apoptosis > Intracellular > Associated Proteins
Signal Transduction > Adapters > Cytoplasmic
Signal Transduction > Growth Factors/Hormones > TNF
Signal Transduction > Signaling Pathway > Nuclear Signaling > NFkB Pathway
SWISS
Gene ID
保存条件
Store at 4℃ for short term. Store at -20℃ for long term. Avoid repeated freeze/thaw cycles.
注意事项
This product as supplied is intended for research use only, not for use in human, therapeutic or diagnostic applications.
数据库链接
产品介绍
信号传导(Signaling Intermediates)
TRAF2在介导细胞凋亡信号及在肿瘤细胞的凋亡中发挥着一定的作用。TRAF2分布较广泛,在所有检测的组织中都有表达,包括心脏、脑、脾脏、肺、肝、骨骼肌、肾和睾丸等,TRAF2可以介导TNFRII、CD40、CD30和EV病毒转化蛋白LMP1活化NF-kB。
TRAF2在介导细胞凋亡信号及在肿瘤细胞的凋亡中发挥着一定的作用。TRAF2分布较广泛,在所有检测的组织中都有表达,包括心脏、脑、脾脏、肺、肝、骨骼肌、肾和睾丸等,TRAF2可以介导TNFRII、CD40、CD30和EV病毒转化蛋白LMP1活化NF-kB。
背景资料
Regulates activation of NF-kappa-B and JNK and plays a central role in the regulation of cell survival and apoptosis.

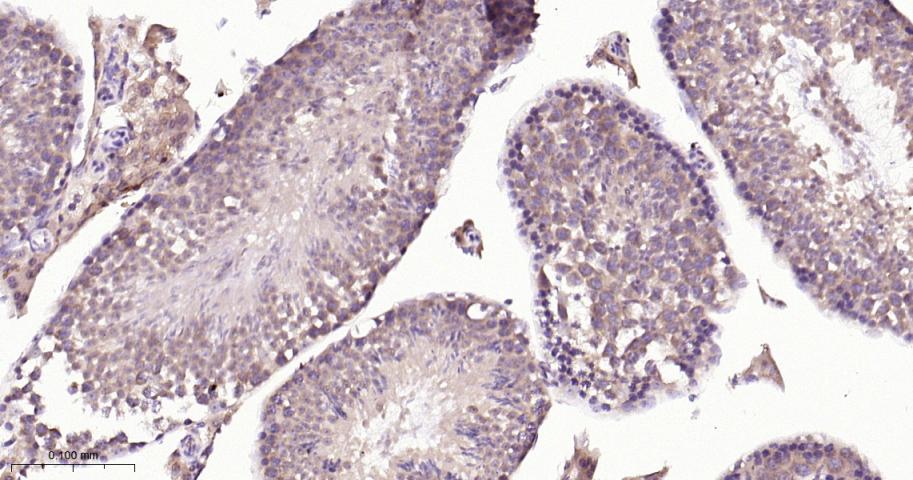
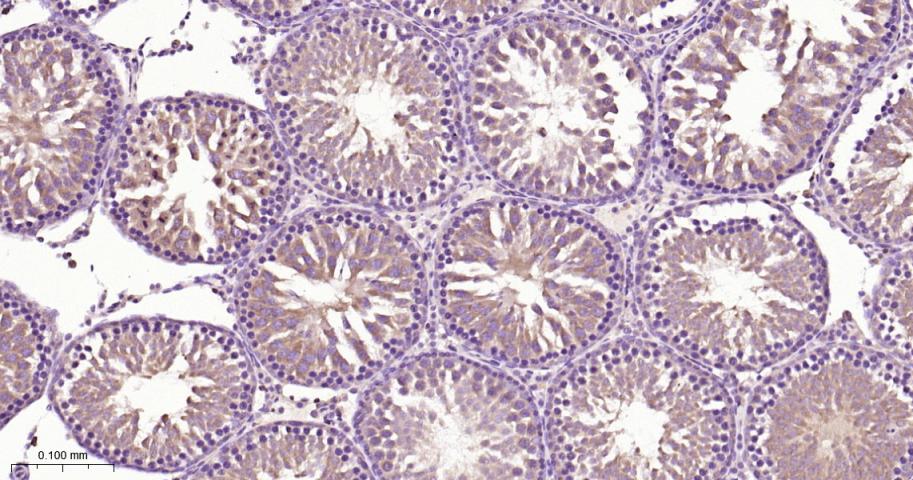
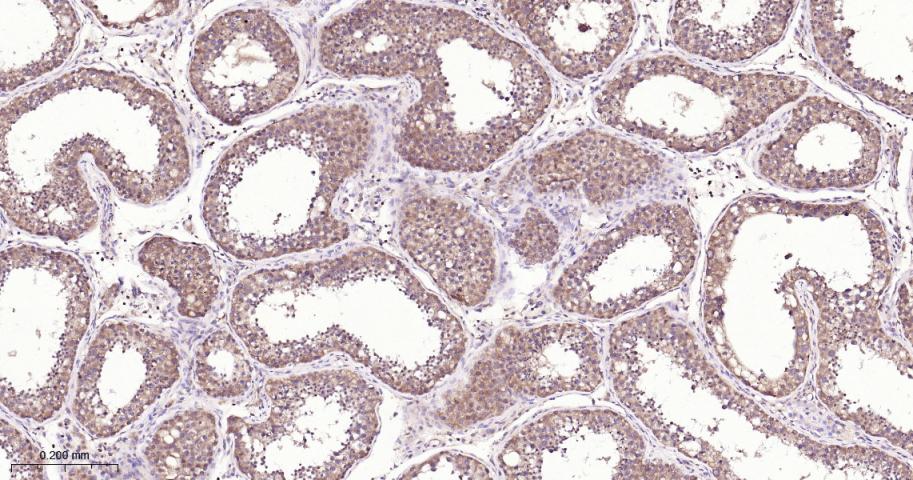
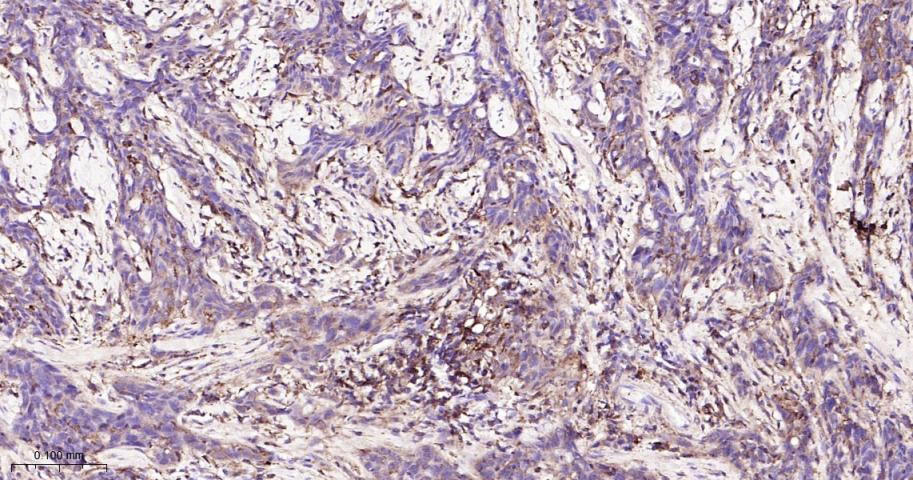
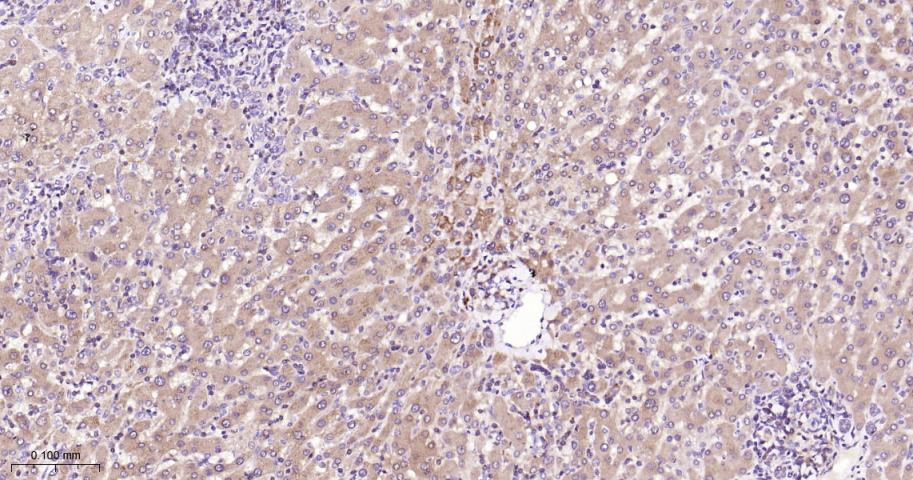
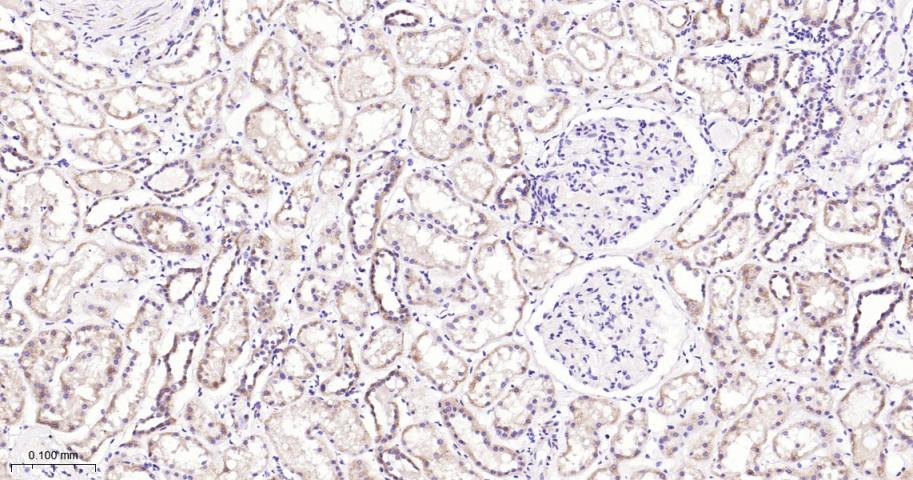
产品应用
| 应用 | 已检合格种属 | 预测种属 | 推荐稀释比例 |
|---|---|---|---|
| WB | Human | Mouse, Rat | 1:500-2000 |
| IHC-P | Human, Mouse, Rat | 1:50-200 | |
| IHC-F | Human, Mouse, Rat | 1:50-200 | |
| IF | Human, Mouse, Rat | 1:50-200 | |
| Flow-Cyt | Human, Mouse, Rat | 1:50-100 | |
| ICC/IF | Human, Mouse, Rat | 1:50-200 | |
| IP | Human, Mouse, Rat | 1:20-50 |
交叉反应
交叉反应: Human, Mouse, Rat
相关产品
暂无相关产品
靶标
基因名
TRAF2
蛋白名
TNF receptor-associated factor 2
亚基
Homotrimer, and heterotrimer with TRAF1 and TRAF3 (via TRAF domain). The domain containing the RING-type and the first TRAF-type zinc finger can also form homodimers (in vitro). Interacts with TNFRSF1B/TNFR2, TNFRSF4, TNFRSF5/CD40, CD27/TNFRSF7, TNFRSF8/CD30, TNFRSF9/CD137, TNFRSF11A/RANK, TNFRSF13B/TACI, TNFRSF14, TNFRSF16/NGFR, TNFRSF17/BCMA, TNFRSF18/AITR, TNFRSF19/TROY, TNFRSF19L/RELT, XEDAR, EDAR, Epstein-Barr virus BNFL1/LMP-1 and IL15RA. Interacts with CDK9, CSK, MAP3K1, MAP3K5, MAP3K11, MAP3K14, MAP4K2, RIPK1, RIPK2, TNIK, TBK1, SPHK1, TRADD, TRAFD1, TRAIP, TANK/ITRAF, TNFAIP3, TDP2, MAVS/IPS1, TICAM1 and TRPC4AP. Interacts with CASP8AP2, NFATC2IP, PEG3 and HIVEP3. Interacts with BIRC2 and BIRC3 N-terminus; a single BIRC2 or BIRC3 molecule interacts with a heterotrimer formed by TRAF1 and TRAF2, or a TRAF2 homotrimer. Identified in a complex composed of TRAF2, TRAF3, BIRC2 and/or BIRC3. Interaction with BIRC2 and/or BIRC3 is essential for degradation of NFKBIA and activation of NF-kappa-B. Interacts with CYLD, USP48, IKKA and IKKB. Interacts (via 'Lys-63'-linked polyubiquitin chains) with TAB2 and TAB3 (By similarity). Interaction with TAOK3 is facilitated under ER stress conditions, such as treatment with tunicamycin, and may promote TRAF2 phosphorylation (By similarity). Interacts with CASP12 under resting conditions; this interaction is reduced in ER stress conditions. Interacts (via zinc fingers) with DAB2IP (via C-terminus PER domain); the interaction occurs in a TNF-alpha-dependent manner (By similarity). Interacts with ERN1; the interaction requires DAB2IP. Interacts with DAB2IP. Interacts (via C-terminus) with EIF2AK2/PKR (via the kinase catalytic domain) (By similarity).
亚细胞定位
Cytoplasm.
组织特异性
isoform 1 and isoform 2 are expressed in spleen, adipose tissues, skeletal muscles, thymus, testis, heart, lung, brain. Isoform 2 is very weakly expressed in heart, lung and brain.
翻译后修饰
Phosphorylated at several serine residues within the first 128 amino acid residues. Phosphorylated at Thr-117 in response to signaling via TNF and TNFRSF1A. Phosphorylation at Thr-117 is required for 'Lys-63'-linked polyubiquitination, but not for 'Lys-48'-linked polyubiquitination. Phosphorylation at Thr-117 is important for interaction with IKKA and IKKB, activation of IKK and subsequent activation of NF-kappa-B (By similarity).
Undergoes both 'Lys-48'-linked and 'Lys-63'-linked polyubiquitination. Polyubiquitinated via 'Lys-63'-linked ubiquitin in response to TNF signaling; this requires prior phosphorylation at Thr-117. 'Lys-63'-linked polyubiquitination promotes TRAF2-mediated activation of NF-kappa-B. Can be polyubiquitinated at several Lys residues via 'Lys-48'-linked ubiquitin chains in response to TNF signaling, leading to proteasomal degradation. Autoubiquitinated, leading to its subsequent proteasomal degradation. Polyubiquitinated by BIRC2 and SIAH2, leading to its subsequent proteasomal degradation. Not ubiquitinated by BIRC3 or SIAH1. Deubiquitinated by CYLD, a protease that specifically cleaves 'Lys-63'-linked polyubiquitin chains (By similarity).
Undergoes both 'Lys-48'-linked and 'Lys-63'-linked polyubiquitination. Polyubiquitinated via 'Lys-63'-linked ubiquitin in response to TNF signaling; this requires prior phosphorylation at Thr-117. 'Lys-63'-linked polyubiquitination promotes TRAF2-mediated activation of NF-kappa-B. Can be polyubiquitinated at several Lys residues via 'Lys-48'-linked ubiquitin chains in response to TNF signaling, leading to proteasomal degradation. Autoubiquitinated, leading to its subsequent proteasomal degradation. Polyubiquitinated by BIRC2 and SIAH2, leading to its subsequent proteasomal degradation. Not ubiquitinated by BIRC3 or SIAH1. Deubiquitinated by CYLD, a protease that specifically cleaves 'Lys-63'-linked polyubiquitin chains (By similarity).
相似性
Belongs to the TNF receptor-associated factor family. A subfamily.
Contains 1 MATH domain.
Contains 1 RING-type zinc finger.
Contains 2 TRAF-type zinc fingers.
Contains 1 MATH domain.
Contains 1 RING-type zinc finger.
Contains 2 TRAF-type zinc fingers.
功能
Regulates activation of NF-kappa-B and JNK and plays a central role in the regulation of cell survival and apoptosis. Required for normal antibody isotype switching from IgM to IgG. Has E3 ubiquitin-protein ligase activity and promotes 'Lys-63'-linked ubiquitination of target proteins, such as BIRC3, RIPK1 and TICAM1. Is an essential constituent of several E3 ubiquitin-protein ligase complexes, where it promotes the ubiquitination of target proteins by bringing them into contact with other E3 ubiquitin ligases. Regulates BIRC2 and BIRC3 protein levels by inhibiting their autoubiquitination and subsequent degradation; this does not depend on the TRAF2 RING-type zinc finger domain. Isoform 2 does not seem to mediate activation of NF-kappa-B but inhibits isoform 1 activity. Plays a role in mediating activation of NF-kappa-B by EIF2AK2/PKR.
标记抗体
暂无标记数据
同靶标产品
暂无同靶标产品
相关文献
提示: 发表研究结果有使用 bsm-62996R 时请让我们知道,以便我们可以引用参考文章。作为回馈,资料提供者将获得我们送上的小礼品。
暂无相关文献
常见问题
暂无常见问题