白介素-1受体相关激酶4重组兔单抗
Rrmab?兔单抗
货号:bsm-61520R
产品详情
相关标记
相关产品
相关文献
常见问题
概述
产品编号
bsm-61520R
产品类型
重组兔单抗
英文名称
IRAK4 Recombinant Rabbit mAb
中文名称
白介素-1受体相关激酶4重组兔单抗
英文别名
IMD67; IPD1; IRAK-4; NY-REN-64; REN64; 8430405M07Rik; 9330209D03Rik; IRAK4_HUMAN; IRAK4; Renal carcinoma antigen NY-REN-64; 2.7.11.1; IRAK4_MOUSE;
抗体来源
Rabbit
免疫原
A synthesized peptide derived from human IRAK4: 1-64
亚型
IgG
性状
Liquid
纯化方法
affinity purified by Protein A
克隆类型
Recombinant
克隆号
19A20
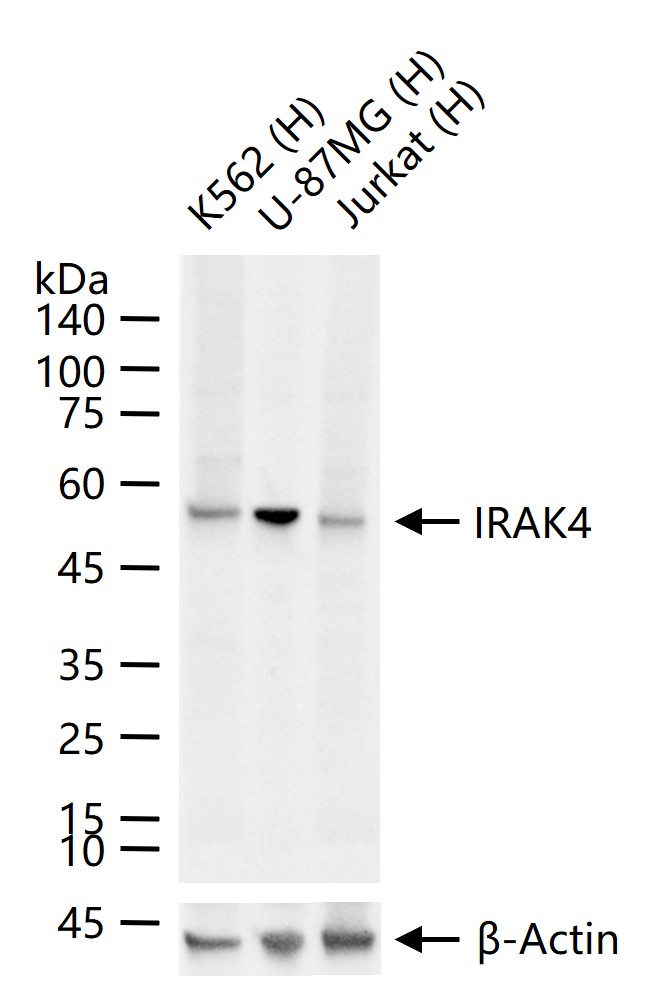
理论分子量
52 kDa
检测分子量
52 kDa
储存液
10mM phosphate buffered saline(pH 7.4) with 150mM sodium chloride, 0.05% BSA, 0.02% Proclin300 and 50% glycerol.
研究领域
SWISS
Gene ID
保存条件
Store at 4℃ for short term. Store at -20℃ for long term. Avoid repeated freeze/thaw cycles.
注意事项
This product as supplied is intended for research use only, not for use in human, therapeutic or diagnostic applications.
数据库链接
背景资料
Serine/threonine-protein kinase that plays a critical role in initiating innate immune response against foreign pathogens. Involved in Toll-like receptor (TLR) and IL-1R signaling pathways.

产品应用
| 应用 | 已检合格种属 | 预测种属 | 推荐稀释比例 |
|---|---|---|---|
| WB | Human | 1:1000-2000 |
交叉反应
交叉反应: Human
相关产品
暂无相关产品
靶标
基因名
IRAK4
蛋白名
Interleukin-1 receptor-associated kinase 4
亚基
Associates with MYD88 and IRAK2 to form a ternary complex called the Myddosome. Once phosphorylated, IRAK4 dissociates from the receptor complex and then associates with the TNF receptor-associated factor 6 (TRAF6), IRAK1, and PELI1; this intermediate complex is required for subsequent NF-kappa-B activation. Interacts with IL1RL1.
亚细胞定位
Cytoplasm.
翻译后修饰
Phosphorylated.
疾病
Defects in IRAK4 are the cause of recurrent isolated invasive pneumococcal disease type 1 (IPD1) [MIM:610799]. Recurrent invasive pneumococcal disease (IPD) is defined as two episodes of IPD occurring at least 1 month apart, whether caused by the same or different serotypes or strains. Recurrent IPD occurs in at least 2% of patients in most series, making IPD the most important known risk factor for subsequent IPD.
Defects in IRAK4 are the cause of IRAK4 deficiency (IRAK4D) [MIM:607676]. IRAK4 deficiency causes extracellular pyogenic bacterial and fungal infections in otherwise healthy children.
Defects in IRAK4 are the cause of IRAK4 deficiency (IRAK4D) [MIM:607676]. IRAK4 deficiency causes extracellular pyogenic bacterial and fungal infections in otherwise healthy children.
相似性
Belongs to the protein kinase superfamily. TKL Ser/Thr protein kinase family. Pelle subfamily.
Contains 1 death domain.
Contains 1 protein kinase domain.
Contains 1 death domain.
Contains 1 protein kinase domain.
功能
Serine/threonine-protein kinase that plays a critical role in initiating innate immune response against foreign pathogens. Involved in Toll-like receptor (TLR) and IL-1R signaling pathways. Is rapidly recruited by MYD88 to the receptor-signaling complex upon TLR activation to form the Myddosome together with IRAK2. Phosphorylates initially IRAK1, thus stimulating the kinase activity and intensive autophosphorylation of IRAK1. Phosphorylates E3 ubiquitin ligases Pellino proteins (PELI1, PELI2 and PELI3) to promote pellino-mediated polyubiquitination of IRAK1. Then, the ubiquitin-binding domain of IKBKG/NEMO binds to polyubiquitinated IRAK1 bringing together the IRAK1-MAP3K7/TAK1-TRAF6 complex and the NEMO-IKKA-IKKB complex. In turn, MAP3K7/TAK1 activates IKKs (CHUK/IKKA and IKBKB/IKKB) leading to NF-kappa-B nuclear translocation and activation. Alternatively, phosphorylates TIRAP to promote its ubiquitination and subsequent degradation. Phosphorylates NCF1 and regulates NADPH oxidase activation after LPS stimulation suggesting a similar mechanism during microbial infections.
标记抗体
暂无标记数据
同靶标产品
暂无同靶标产品
相关文献
提示: 发表研究结果有使用 bsm-61520R 时请让我们知道,以便我们可以引用参考文章。作为回馈,资料提供者将获得我们送上的小礼品。
暂无相关文献
常见问题
暂无常见问题