ATP依赖解旋酶SMARCA2重组兔单抗
Rrmab?兔单抗
货号:bsm-60621R
产品详情
相关标记
相关产品
相关文献
常见问题
概述
产品编号
bsm-60621R
产品类型
重组兔单抗
英文名称
SMARCA2/BRM Recombinant Rabbit mAb
中文名称
ATP依赖解旋酶SMARCA2重组兔单抗
英文别名
BAF190; BIS; BRM; NCBRS; SAMRCA2; SNF2; SNF2L2; SNF2LA; SWI2; Sth1p; hBRM; hSNF2a; 2610209L14Rik; SNF2alpha; brahma; SMCA2_HUMAN; SMARCA2; BRG1-associated factor 190B (BAF190B); Probable global transcription activator SNF2L2; Protein brahma homolog (hBRM); SNF2-alpha; 3.6.4.-; BAF190B; SNF2A; SMCA2_MOUSE; Protein brahma homolog;
抗体来源
Rabbit
亚型
IgG
性状
Liquid
纯化方法
affinity purified by Protein A
克隆类型
Recombinant
克隆号
E7F8
浓度
1mg/ml
储存液
0.01M TBS (pH7.4) with 1% BSA, 0.02% Proclin300 and 50% Glycerol.
研究领域
保存条件
Shipped at 4℃. Store at -20℃ for one year. Avoid repeated freeze/thaw cycles.
注意事项
This product as supplied is intended for research use only, not for use in human, therapeutic or diagnostic applications.
数据库链接
背景资料
A transcriptional coactivator cooperating with nuclear hormone receptors to potentiate transcriptional activation. SMARCA2 / BRM belongs to the SNF2/RAD54 helicase family, is a homologue of the Saccharomyces cerevisiae SWI2/SNF2 and Drosophila brahma proteins. It contains a methyl lysine containing bromo domain and an HSA domain. The yeast protein SNF2, also known as SWI2, is involved in transcriptional activation of numerous genes. It contains a domain that is highly conserved among several known helicases and is required for transcriptional activity. SNF2/SWI2 is highly homologous to the Drosophila protein 'brahma' (brm). Although the 2 proteins show nuclear localization during interphase, they are excluded from the condensed chromosomes during mitosis. They found that the level of BRM, but not BRG1, was strongly reduced during mitosis. Phosphorylation of hbrm and BRG1 did not disrupt their association with SNF5 but correlated with a decreased affinity for the nuclear structure in early M phase.

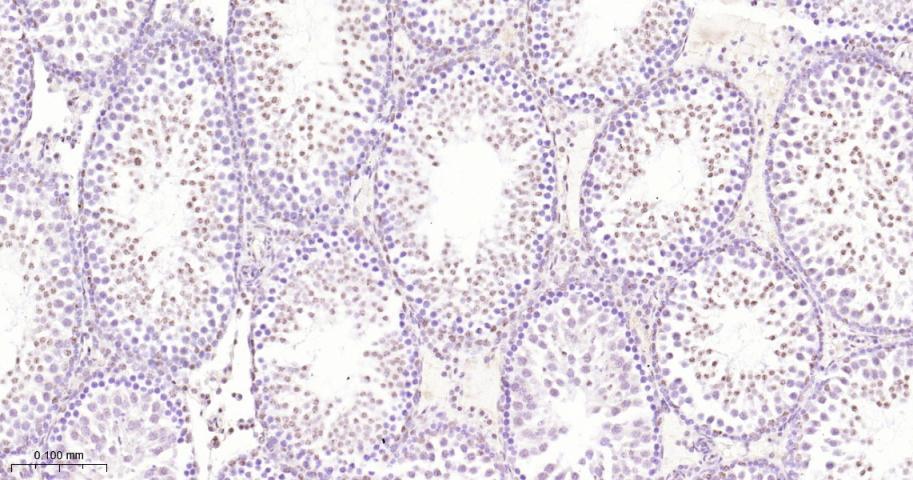
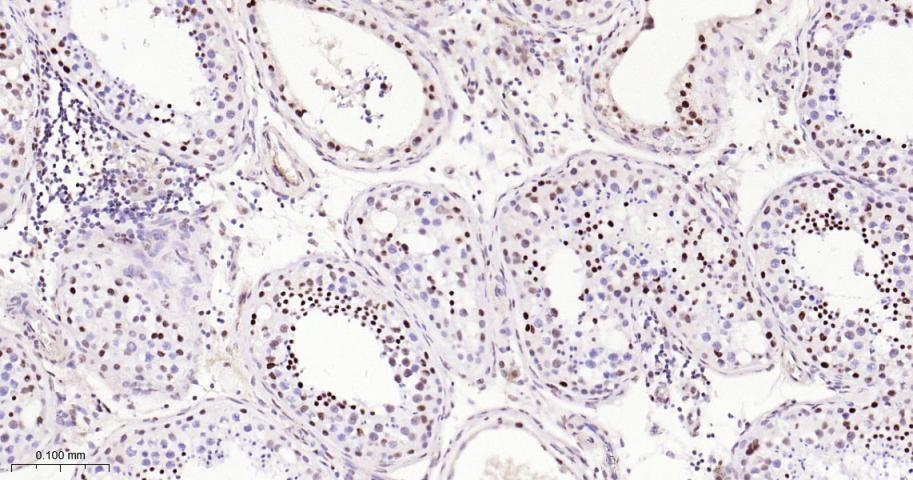
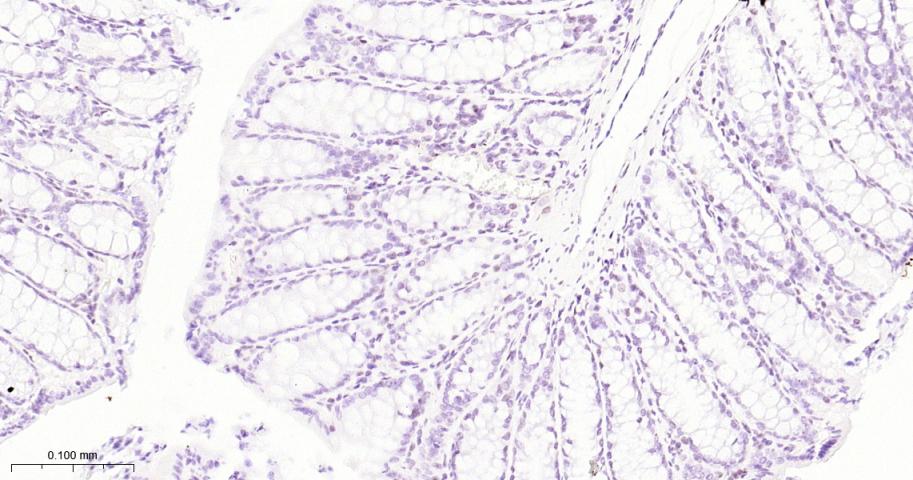
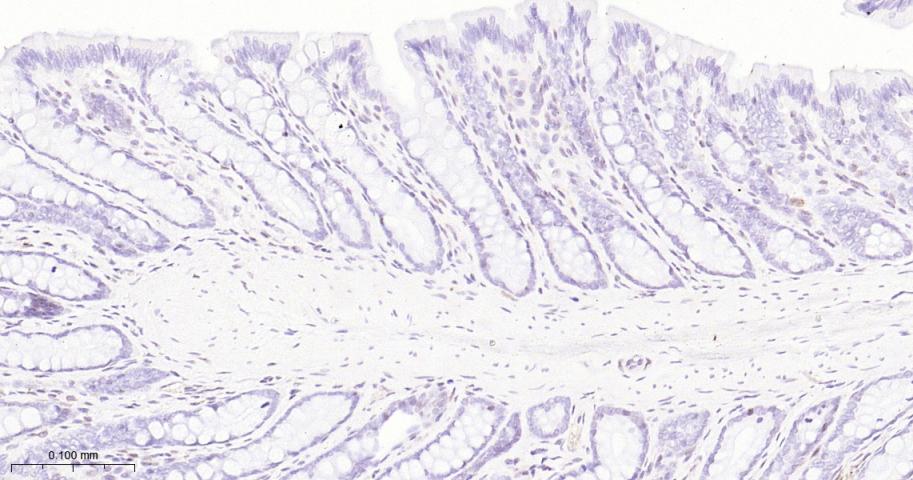
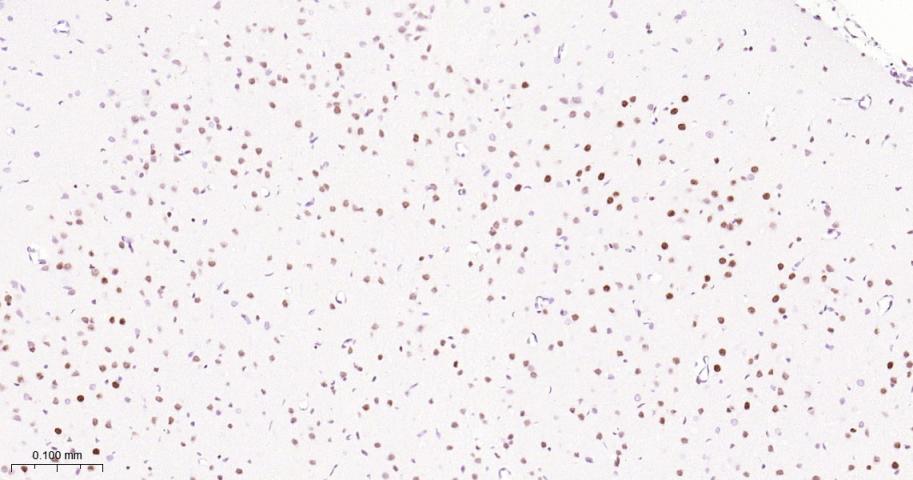
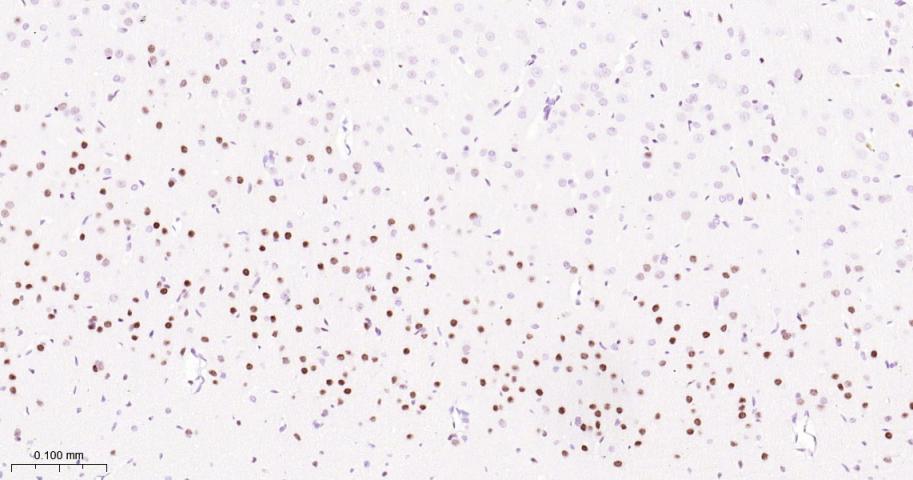
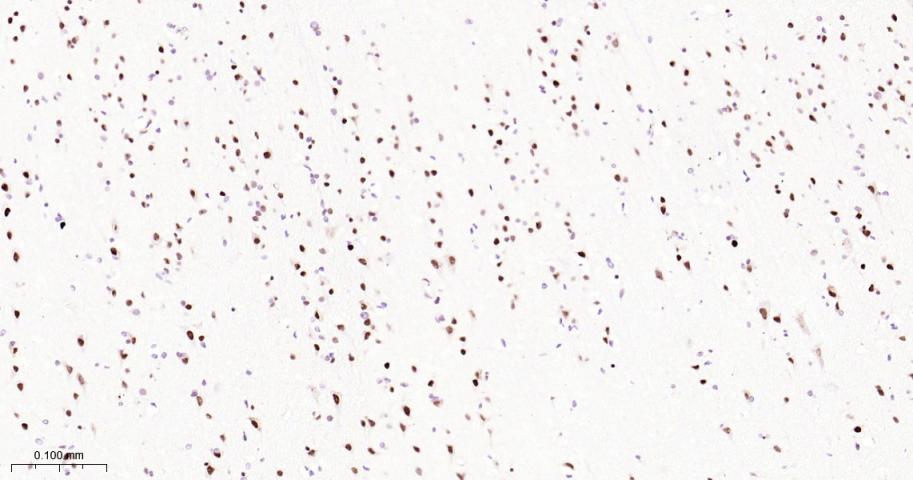
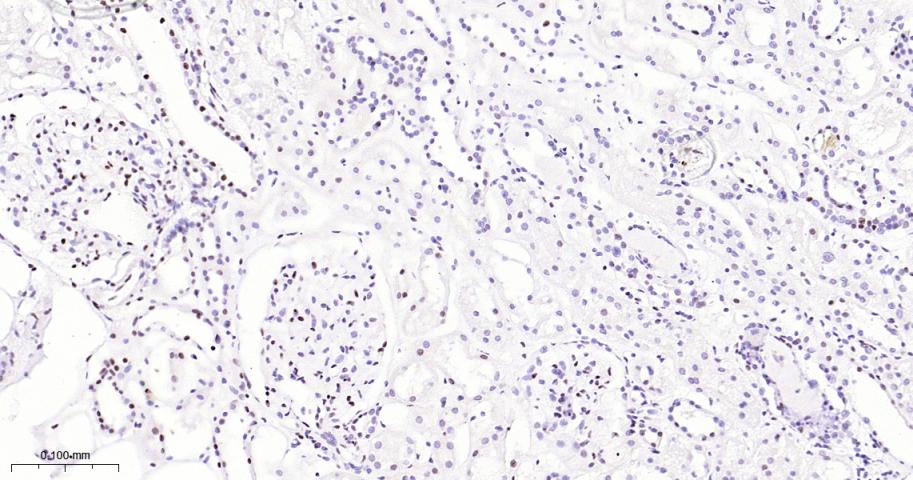
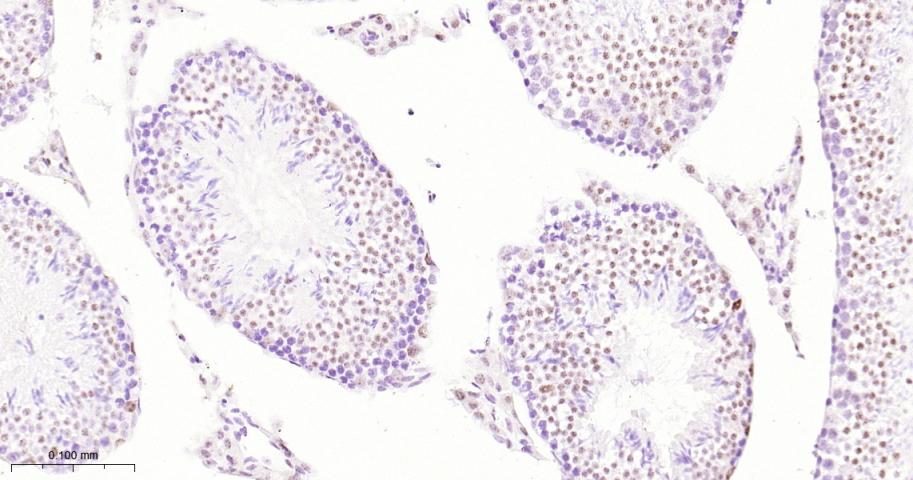
产品应用
| 应用 | 已检合格种属 | 预测种属 | 推荐稀释比例 |
|---|---|---|---|
| IHC-P | Human, Mouse, Rat | 1:50-200 | |
| IHC-F | Human, Mouse, Rat | 1:50-200 | |
| IF | Human, Mouse, Rat | 1:50-200 |
交叉反应
交叉反应: Human, Mouse, Rat
相关产品
暂无相关产品
靶标
基因名
SMARCA2
蛋白名
Probable global transcription activator SNF2L2
亚基
Component of the BAF complex.
亚细胞定位
Nucleus.
翻译后修饰
Phosphorylated upon DNA damage, probably by ATM or ATR.
相似性
Belongs to the SNF2/RAD54 helicase family.
Contains 1 bromo domain.
Contains 1 helicase ATP-binding domain.
Contains 1 helicase C-terminal domain.
Contains 1 HSA domain.
Contains 1 bromo domain.
Contains 1 helicase ATP-binding domain.
Contains 1 helicase C-terminal domain.
Contains 1 HSA domain.
功能
Transcriptional coactivator cooperating with nuclear hormone receptors to potentiate transcriptional activation. Also involved in vitamin D-coupled transcription regulation via its association with the WINAC complex, a chromatin-remodeling complex recruited by vitamin D receptor (VDR), which is required for the ligand-bound VDR-mediated transrepression of the CYP27B1 gene. Belongs to the neural progenitors-specific chromatin remodeling complex (npBAF complex) and the neuron-specific chromatin remodeling complex (nBAF complex). During neural development a switch from a stem/progenitor to a post-mitotic chromatin remodeling mechanism occurs as neurons exit the cell cycle and become committed to their adult state. The transition from proliferating neural stem/progenitor cells to post-mitotic neurons requires a switch in subunit composition of the npBAF and nBAF complexes. As neural progenitors exit mitosis and differentiate into neurons, npBAF complexes which contain ACTL6A/BAF53A and PHF10/BAF45A, are exchanged for homologous alternative ACTL6B/BAF53B and DPF1/BAF45B or DPF3/BAF45C subunits in neuron-specific complexes (nBAF). The npBAF complex is essential for the self-renewal/proliferative capacity of the multipotent neural stem cells. The nBAF complex along with CREST plays a role regulating the activity of genes essential for dendrite growth (By similarity).
标记抗体
暂无标记数据
同靶标产品
暂无同靶标产品
相关文献
提示: 发表研究结果有使用 bsm-60621R 时请让我们知道,以便我们可以引用参考文章。作为回馈,资料提供者将获得我们送上的小礼品。
暂无相关文献
常见问题
暂无常见问题